Dott. Giannantonio Spena
Director of Neurosurgery Unit
Fondazione IRCCS Policlinico San Matteo
Pavia, Italy
Fondazione IRCCS Policlinico San Matteo
Pad. Nuovo Ospedale "DEA"
Piano +7 - Corpo A
Secretary + 39 382 502780
For Private Outpatient Appointment
Policlinico San Matteo: you can book an appointment by calling +39 382 501788 or by filling out the online form.
- Poliambulatorio Akesis viale Libertà 4, Pavia
+39 382 302996

Cerebral gliomas are rare and highly heterogeneous tumors that originate from the cells that make up the cerebral cortex and the white matter.
By their very nature, they tend to infiltrate the brain, blending with it, although in some cases they can be clearly distinguished from healthy brain tissue. This infiltrative behavior makes surgical removal particularly complex, and surgery alone rarely ensures a cure. Moreover, gliomas often infiltrate areas of the brain that are essential for verbal expression, reading, writing, motor coordination, and body sensation.
Traditionally, certain areas of the brain are defined as “eloquent” (that is, highly functional), while others are considered non-eloquent. In reality, all brain areas have functions — the difference lies in the fact that some regions act as major hubs where countless neural connections converge. Damage to one of these hubs can therefore cause neurological deficits that are difficult to recover from. Other areas are less interconnected, and their functions can, over time, be taken over by other brain regions.
Another crucial characteristic of gliomas is the wide variability in their growth rate. Some grow very slowly, without significantly affecting the patient’s brain functions, and often the patient becomes aware of the tumor only when it reaches a considerable size (see images below). When, on the other hand, the growth rate is high, it is more likely that the patient will experience symptoms related to the malfunction of the brain area irritated by the tumor’s presence.
One of the main goals of neurosurgery is to achieve the most extensive possible tumor removal while minimizing damage to brain functions. It has been clearly demonstrated that the extent of resection correlates with longer survival.
Because anatomical variability and the distribution of functions in the cortex and white matter are enormous from one person to another, it is impossible to predict with absolute precision whether an area infiltrated by the tumor can be safely removed — and to what extent its removal would produce permanent neurological deficits.
Fortunately, today there are diagnostic technologies that allow visualization of the activity of specific brain regions and help determine whether they are involved in a particular function. The most commonly used test for this purpose is Functional Magnetic Resonance Imaging (fMRI), which shows, in the form of colored “spots,” the areas of the cerebral cortex that are activated while the subject performs certain tasks.
Similarly, but through different techniques, it is possible to reconstruct a model of the connections between neurons (at least the larger fiber bundles). Certain MRI sequences exploit the diffusion of water molecules along neural connections to produce highly reliable 3D images that can help the surgeon understand the relationship between the tumor and the white matter tracts.
Despite these major advances in neuroradiology, there are still limitations that prevent surgeons from relying solely on these methods when operating on brain tumors located in critical areas.
For this reason, brain mapping through direct cortical stimulation in awake patients, along with intraoperative monitoring, has become the gold standard for brain tumor surgery in critical areas.
The purpose of applying a non-damaging electric current to the cerebral cortex or white matter is to excite or inhibit groups of neurons, temporarily creating a short circuit that simulates what would happen if those neurons were removed. In this way, a functional map of the exposed brain area can be drawn.
Using this technique, it is possible to study various brain functions such as sensory processing, verbal language, writing, reading, calculation, and vision.
Sensory and motor areas can also be mapped while the patient is asleep. The choice of which technique to use is discussed with the surgeon on a case-by-case basis. Conversely, mapping of the other functions mentioned above requires the patient’s full cooperation during the surgical procedure.
BRAIN GLIOMAS
Useful links
Classification of brain tumors
according to the World Health Organization (WHO 2021 )
In-depth analysis
Historical notes
The first description of direct brain stimulation in neurosurgery has been attributed to Victor Horsley and David Ferrier in 1884, followed by William Keen in 1888, Leonard Bidwell and Charles Sherrington in May 1893, and Fedor Krause in November 1893.
Wilder Penfield was the pioneer of direct brain mapping on a conscious patient, aiming to preserve eloquent areas. Thanks to this technique, Penfield was able to describe the organization of the motor and sensory areas of the cerebral cortex. In the 1970s, the technique was refined by George Ojemann, who introduced constant-pulse biphasic current; he also improved the type of intraoperative language testing. In the 1990s, Mitchel Berger applied this technique to oncological neurosurgery and was also the first to apply stimulation to white matter tracts (corticospinal tract). Subsequently, Hugues Duffau extended and codified the indications for direct cortical and subcortical brain stimulation. The technique consisted (and still is) of applying a current via a bipolar electrode to the cerebral cortex.
Subsequently, other stimulation techniques (such as monopolar) and systems for monitoring the effects of the stimulation were developed.
To learn more about this topic, click here
or Click here .
Clinical examples

Magnetic Resonance Imaging with DTI Fiber Tracking
This MRI sequence allows us to reconstruct a model of the white matter bundles (i.e. the connections between neurons)
Magnetic Resonance Imaging with DTI Fiber Tracking
This MRI sequence allows us to reconstruct a model of the white matter bundles (i.e. the connections between neurons)




Some images of surgical interventions performed with mapping on the patient awake
The electrical current for brain stimulation and mapping is applied through a bipolar electrode. The patient is able to perform various tasks such as reading, writing, counting, repeating words, and so on. The small white numbered labels serve to create a map of the brain area to which the stimulation current was applied.
Removal of a large slow-growing glioma of the right fronto-temporo-insular region (electrophysiological monitoring)

Slow-growing glioma
The bottom left shows an MRI of a patient with a large, slow-growing glioma infiltrating the mesial frontal cortex, along with the cingulum and corpus callosum. The patient led a completely normal life, and only one epileptic seizure required hospitalization. Neuropsychological testing revealed normal overall functioning with only some memory problems. The postoperative MRI on the right shows complete removal of the mass; the patient was able to fully resume his daily activities within six weeks. Two years later, he has not had any further epileptic seizures.



The importance of awake surgery and electrophysiological monitoring
Neurosurgery of tumors located in eloquent areas requires techniques and technologies capable of guiding the surgeon during resection, highlighting the presence of areas responsible for neurological functions within or adjacent to the tumor mass.
Below is the case of a patient who had previously undergone surgery at another center for the removal of a glioma in the supplementary motor area, very close to the areas that control body movement. Since the surgery was performed without the techniques discussed above, the tumor was only partially removed. The patient also continued to suffer from numerous epileptic seizures on a weekly basis.
It was therefore decided to perform a second operation, using awake surgery and mapping of cortical and subcortical functions. The removal was complete, and the patient experienced immediate relief from his epileptic seizures.

* The green-bordered areas represent residual tumor not removed during the initial surgery. The lower images show complete resection.

The importance of awake surgery and electrophysiological monitoring
Awake surgery was also crucial in the case reported below. This young woman had experienced several epileptic seizures that led to the diagnosis of frontal lobe tumor. Aside from the seizures, the patient was intact and leading a normal life. She underwent surgery under local anesthesia with cognitive function mapping, resulting in a complete removal. The diagnosis was a WHO II astrocytoma (IDH1 wt, no LOH1p19q). She underwent radiation therapy, and after three and a half years, the patient is leading a normal life without further seizures, and there are currently no signs of the disease returning.



Below is the case of a young patient who underwent surgery for a left frontal astrocytoma four years earlier at another institution. The tumor was not completely removed. The patient continued to suffer from seizures, and follow-up MRIs showed an increase in residual tumor. He therefore underwent awake surgery with mapping of various language functions. On the right is the postoperative MRI confirming complete removal.



The case reported below concerns a young adult with a tumor (midline H3K27M glioma). The tumor invaded the posterior portion (pulvinar) of the right thalamus. Surgery was performed with electrophysiological monitoring (transcranial EPS and PEM; cortical strip for PEM). The removal was complete without any postoperative deficits.

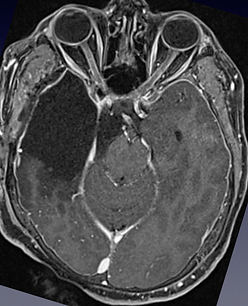
Patient admitted to the emergency room with confusion and drowsiness. The photo on the left shows the preoperative MRI, which demonstrates the presence of a large, suspicious glioma infiltrating the right temporal lobe and invading the ambiens and femoral cisternae. The patient underwent emergency surgery for complete removal of the mass with the aid of electrophysiological monitoring. On the right is the postoperative MRI, which confirms total resection. The diagnosis was IDH 1 wild-type astrocytoma.